VANCOUVER, BC, April 29, 2021 – Valeo Pharma (CSE: VPH) (OTCQB: VPHIF) (FSE: VP2) has reached an agreement with the Ontario Public Drug Program to cover the costs of Redesca® and Redesca HP® – anticoagulants that reduce the risks of blood clots.
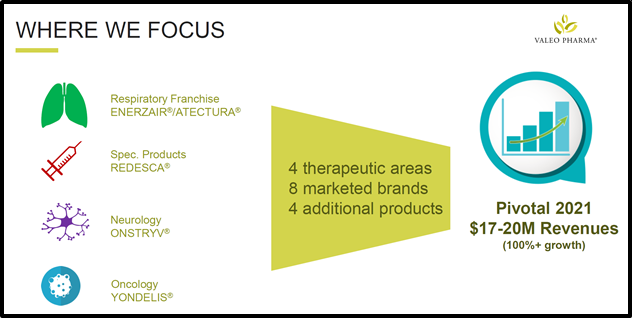
Launched as a public entity in 2019 – Valeo is a specialty pharma company that commercialises prescription products in Canada with a focus on Neurodegenerative Diseases, Oncology and Hospital Specialty Products.
Valeo’s business model side-steps the typical pharma-development pipeline of animal-trials, human-trials and applications for regulatory approval.
VPH partners with pharma companies that have drugs already approved in other jurisdictions, providing those companies with the infrastructure and expertise to navigate the Canadian landscape of drug commercialization.
The Product Listing Agreement (PLA) with the Executive Officer of the Ontario Public Drug Program means that eligible Ontario patients requiring Redesca® and Redesca HP® are insured for the costs of the drugs.
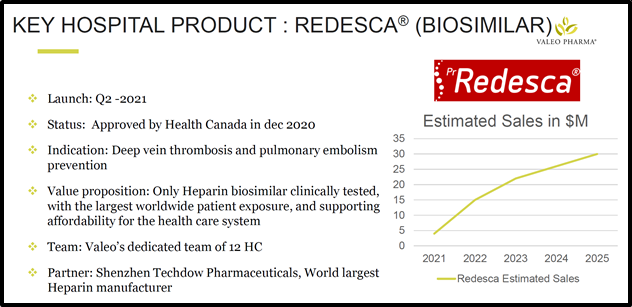
Redesca is a low molecular weight heparin (LMWH) biosimilar.
LMWHs are injectable anticoagulant drugs used primarily to treat and prevent deep vein thrombosis and pulmonary embolism.
Common hospital-associated blood clots:
1. Deep vein thrombosis (DVT): a blood clot that forms in a deep vein, most commonly the leg or pelvis.
2. Pulmonary embolism (PE): a blood clot that becomes dislodged and passes through blood vessels before reaching the lungs.
“Biosimilar drugs are often confused with generic drugs,” explains The Cancer Center, “Both are marketed as cheaper versions of costly name-brand drugs. Generic drugs are identical to the original in chemical composition, biosimilar drugs are “highly similar,” but close enough to accomplish the same therapeutic and clinical result”.
“Roughly 1 out of 10 hospital deaths are related to blood clots in the lungs,” reports the Center for Disease Control (CDC).
Blood clots are a leading cause of preventable hospital death in the United States.
-
About half of all blood clots occur during or within 3 months of a hospital stay or surgery
-
Many of these blood clots can be safely prevented
-
Nearly half of all hospital patients do not receive proper prevention measures
Redesca has already clocked more than 150 million patient days in foreign markets.
“The regulatory approval of Redesca is a significant corporate milestone for Valeo and good news for the Canadian healthcare system,” stated Valeo CEO Steve Saviuk, “Mandatory use of biosimilars is expected to deliver significant savings to provincial healthcare plans.”
On April 27, 2021 VPH announced that it upsized and closed a $6.645 million non-brokered private placement of unsecured non-convertible debenture units.
The Debentures will mature at the latest 9 months after the closing and bear interest at a rate of 8% per annum from the date of issue, payable in cash, semi-annually in arrears.
“I cannot over emphasize what a pivotal year 2021 is becoming with the recent launch of Redesca® and the imminent launches of Enerzair® Breezhaler® and Atectura® Breezhaler®,” added Saviuk.
“With Ontario representing 37% of the Canadian market for LMWHs, the listing of Redesca on the Ontario public formulary is a key milestone for the Redesca commercialization program,” stated Frederic Fasano, President and COO of Valeo.
“This is welcome news for millions of Canadians who rely on public insurance to access their prescription medications,” added Fasano, “and for the Government of Ontario who will benefit from significant savings resulting from the listing of the first LMWH biosimilar”.
VPH anticipates that public insurance coverage for Redesca in other provinces will follow.

