VANCOUVER, BC – Medexus Pharmaceuticals (MDP.V) is a specialty pharmaceuticals company that delivers innovative medicines to under-served segments of the market.
With over 20,000 prescription drug products approved for marketing in North America, the pharmacology space is fiercely competitive. Identifying “underserved markets” requires scientific, regulatory and business savvy.
Medexus’ CEO Ken d’Entremont – a chemist by trade – is the former V.P of business development at Big Pharma company Sanofi, where he led the in-licensing initiatives for Sanofi Canada.
The current focus at MDP includes rheumatology (arthritis/joints/muscles), auto-immune disease (diabetes/MS/lupus), specialty oncology (cancer) and pediatrics (child healthcare).
On December 18, 2020 Medexus announced it has signed an exclusive license agreement with Ethypharm to register and commercialize Triamcinolone Hexacetonide Injectable Suspension 20 mg/mL (TH) in the U.S.
TH is used to treat adults and teenagers suffering from arthritis. The effects last twice as long as competitive products.
This exclusive agreement is expected to address existing supply chain issues, while reducing hospital visits and the need for general anesthetics.
Physically active aging Baby Boomers are plagued by joint pain. Next year, the total number of people older than 65 years will jump to 656 million (about 11% of the population). In California, the over-65 population is increasing at twice the speed of millennials.
According to an Acumen Research Report, the global Joint Pain Injections Market is estimated to grow at CAGR 8% in the next six years, to reach USD $5.7 billion by 2026.
Medexus expects to file for FDA approval of TH within 12-24 months.
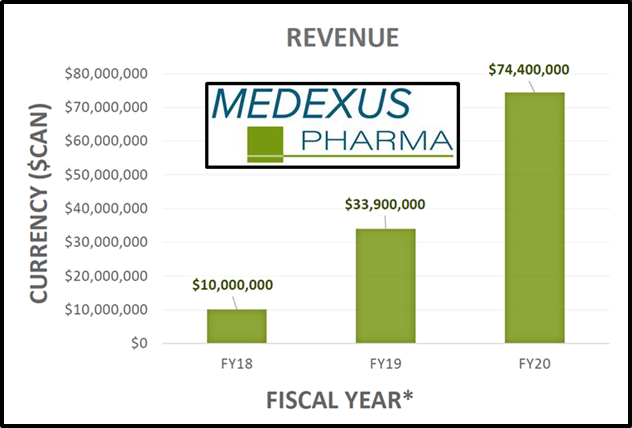
On November 16, 2020 Medexus published financial and operating results for the three months ended September 30, 2020 (Q2, 2021).
Medexus achieved revenue of $23.6 million (CND) for the three-month period ended September 30, 2020, a 40% increase over the same period, the previous year.
The big revenue growth catalyst was the $3 million quarterly revenue derived from IXINITY sales.
IXINITY is used to treat Hemophilia B – a hereditary bleeding disorder. Hemophilia occurs in approximately 1 in 5,000 live births.
IXINITY was absorbed by MDP in February, 2020 in a “transformative acquisition”. At that time, the product was booking 40% year-over-year growth. Since that event, the MDP stock price has risen 74%.
MDP paid USD $30 million for IXINITY. The purchase price was non-dilutive – coming from existing cash and a $20 million credit facility with MidCap Financial.
The U.S. hemophilia B market is approximately USD $734 million and growing, with a highly concentrated prescriber base (limited number of physicians treating the disorder).
“IXINITY is an FDA approved product with a track record of safety, efficacy and growing sales,” stated d’Entremont.
The product is currently approved for patients 12 years and older. An MDP Phase 4 clinical trial is investigating the safety and efficacy of IXINITY for children.
“The pediatric segment is estimated to represent one-third of the hemophilia B population,” stated d’Entremont, who is targeting “a label expansion” for IXINITY.
“Medexus’ $95 million 12-month trailing revenue is within 5% of its current market cap,” stated Global News CEO Guy Bennett on December 20, 2020, “By comparison, the $209 billion Pfizer has 12-month trailing revenue of about $45 billion, about 21% of its market cap”.
Medexus has 43 dedicated North American sales reps.
The November 16, 2020 financials reveal that Medexus’ selling and admin expenses decreased this quarter from 64.4% of revenue to 46.6% of revenue.